
Neutral helium at standard conditions is non-toxic, plays no biological role and is found in trace amounts in human blood. Check oxygen content before entering area. Inhalation risk: On loss of containment this gas can cause suffocation by lowering the oxygen content of the air in confined areas. Health effects of heliumĮffects of exposure: The substance can be absorbed into the body by inhalation. Helium is the 71st most abundant element in the Earth's crust where it is found in 8 parts per billion (10 9). Natural gases contain higher helium concentrations than the atmosphere. There is an about 1000 km layer in the heterosphere at 600 miles where helium is the dominant gas (although the total pressure is very low). Nevertheless, its low molecular weight allows it to escape to space at the same rate of its formation. It could be logical to think that the helium concentration in the atmosphere was higher than it is (5,25 parts per million at sea level). Most of this helium migrates to the surface and enters the atmosphere. Helium is formed in The Earth by natural radioactive decay of heavier elements.
Helium constitutes the 23% of all elemental matter measured by mass. Helium is the second most abundant element in the known universe, after hydrogen. Other applications are its use as pressurizing gas in liquid propellants for rockets, in helium- oxygen mixtures for divers, as working fluid in nuclear reactors cooled down by gas and as gas carrier in chemical analysis by gas chromatography. The main application of ultralow temperature is in the development of the superconductivity state, in which the resistance to the electricity flux is almost zero. Helium is the only cooler which is capable of reaching temperatures lower than 15 K (-434✯). Its biggest potential is found in applications at very low temperatures. The main use of helium is as an inert protection gas in autogenous welding. This application goes on in altitude research and for meteorological balloons. Helium was the first gas used for filling balloons and dirigibles. Helium has many unique properties: low boiling point, low density, low solubility, high thermal conductivity and inertness, so it is use for any application which can explioit these properties. Helium can be liquefied, but its condensation temperature is the lowest among all the known substances. The thermic conductivity and the caloric content are exceptionally high. The density and viscosity of helium vapour are very low. It’s the less reactive element and doesn’t essentially form chemical compounds. It’s less soluble in water than any other gas. Helium is a colourless, odourless, insipid and non-toxic gas.
#ATOMIC MASS OF HELIUM SERIES#
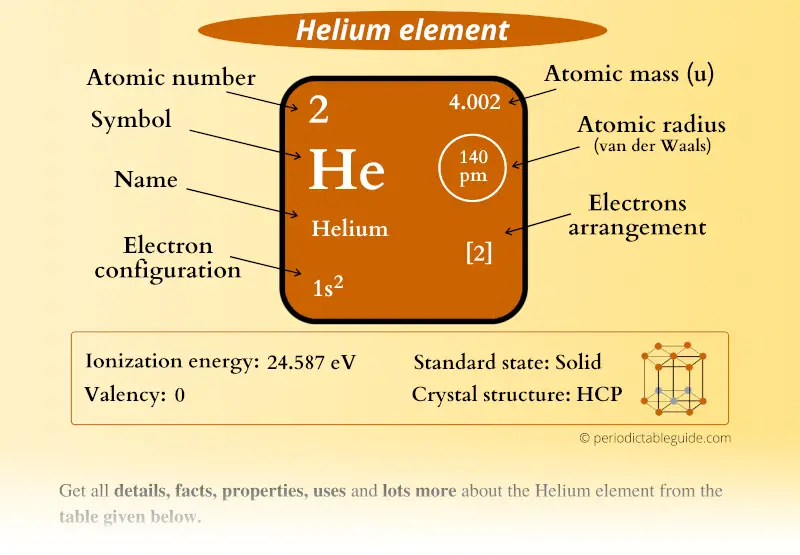
The main helium source in the world is a series of fields of natural gas in the United States. Helium is one of the noble gases of group O in the periodic table. Gaseous chemical element, symbol: He, atomic number: 2 and atomic weight 4,0026 g/mol. Using atomic mass units, what will the binding energy of. Helium - He Chemical properties of helium - Health effects of helium The atomic mass of helium He is 4.0026 u, and the atomic mass of hydrogen H is 1.0078 u.



 0 kommentar(er)
0 kommentar(er)
